First Principle Kinetic Studies of Zeolite-Catalyzed Methylation Reactions
V. Van Speybroeck, J. Van der Mynsbrugge, M. Vandichel, K. Hemelsoet, D. Lesthaeghe, A. Ghysels, G. B. Marin, M. Waroquier, Journal of the American Chemical Society 2011 133 (4), 888-899.
Abstract
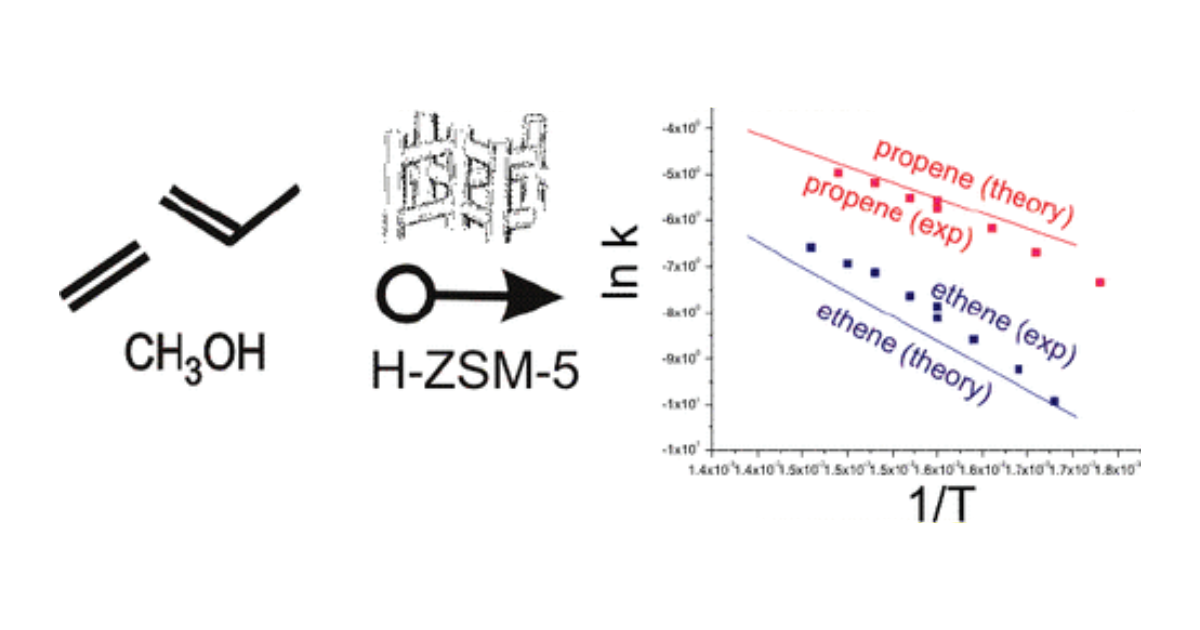
Methylations of ethene, propene, and butene by methanol over the acidic microporous H-ZSM-5 catalyst are studied by means of state of the art computational techniques, to derive Arrhenius plots and rate constants from first principles that can directly be compared with the experimental data. For these key elementary reactions in the methanol to hydrocarbons (MTH) process, direct kinetic data became available only recently [J. Catal. 2005, 224, 115−123; J. Catal. 2005, 234, 385−400]. At 350 °C, apparent activation energies of 103, 69, and 45 kJ/mol and rate constants of 2.6 × 10−4, 4.5 × 10−3, and 1.3 × 10−2 mol/(g h mbar) for ethene, propene, and butene were derived, giving following relative ratios for methylation kethene/kpropene/kbutene = 1:17:50. In this work, rate constants including pre-exponential factors are calculated which give very good agreement with the experimental data: apparent activation energies of 94, 62, and 37 kJ/mol for ethene, propene, and butene are found, and relative ratios of methylation kethene/kpropene/kbutene = 1:23:763. The entropies of gas phase alkenes are underestimated in the harmonic oscillator approximation due to the occurrence of internal rotations. These low vibrational modes were substituted by manually constructed partition functions. Overall, the absolute reaction rates can be calculated with near chemical accuracy, and qualitative trends are very well reproduced. In addition, the proposed scheme is computationally very efficient and constitutes significant progress in kinetic modeling of reactions in heterogeneous catalysis.