Experimental and Theoretical Studies of Pd Cation Reduction and Oxidation During NO Adsorption on and Desorption from Pd/H–CHA
P. Kim, J. Van der Mynsbrugge, M. Head-Gordon, A. T. Bell, The Journal of Physical Chemistry C 2022 126 (44), 18744-18753.
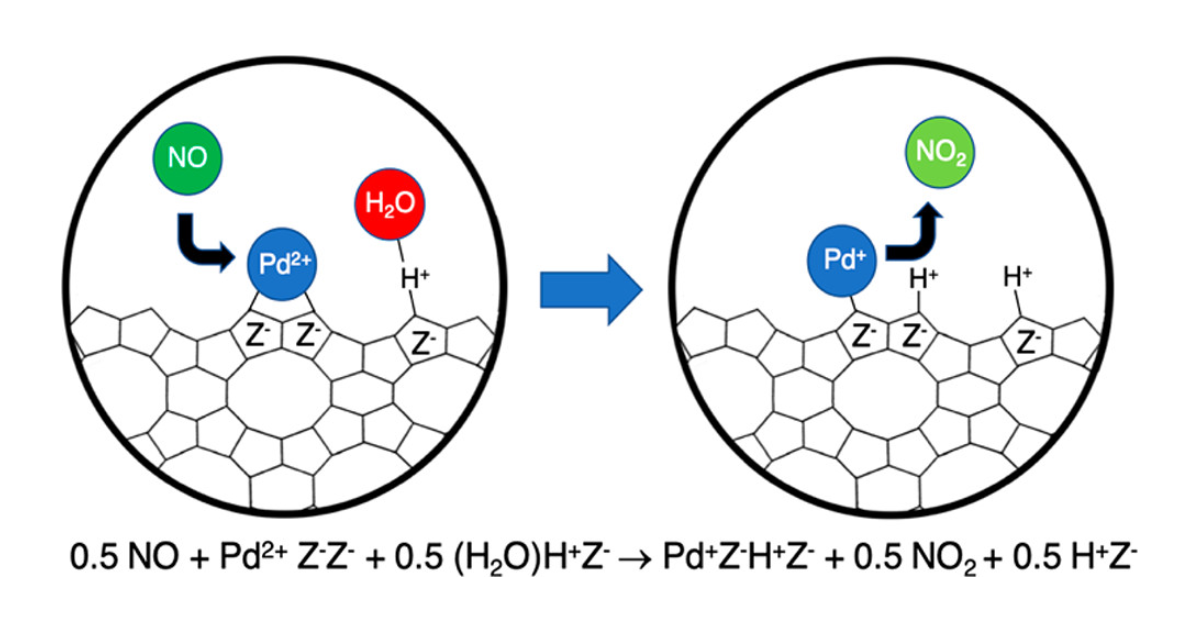
Abstract
Passive NOx adsorbers (PNAs) have been proposed for trapping NOx present in automotive exhaust during the period of cold start during which the three-way convertor is not yet hot enough to be effective for NOx reduction. Pd-exchanged chabazite (Pd/H–CHA) is a good candidate for passive NOx adsorption due to its ability to store NO and retain it to high temperatures (>473 K). Previous research suggests that NO adsorbs on both Pd2+ and Pd+ cations and that NO desorption from Pd2+ cations occurs at lower temperatures than from Pd+ cations. Since experimental evidence shows that Pd exchanges into CHA exclusively as Pd2+, it is not clear how these cations are reduced to Pd+. In this study we show through experiments and theoretical analysis that Pd+ cations can form via two processes, each of which involves water adsorbed on Brønsted-acid sites of the zeolite. The first of these processes is 1.5 NO + Pd2+Z–Z– + 0.5 (H2O)H+Z– → (NO)Pd+Z–H+Z– + 0.5 NO2 + 0.5 H+Z–. Experiments confirm that the ratio of the NO2 formed upon NO adsorption to the NO desorbing from Pd+ at elevated temperatures corresponds to 0.5. Pd2+ can also be reduced via the reaction 1.5 CO + Pd2+Z–Z– + 0.5 (H2O)H+Z– → (CO)Pd+Z–H+Z– + 0.5 CO2 + 0.5 H+Z–. Upon subsequent adsorption of NO, NO fully displaces CO from Pd+ to form (NO)Pd+Z–H+Z–. In this case, the amount of CO2 formed upon CO adsorption is 0.5 of the NO desorbing at elevated temperatures from Pd+. Gibbs free energy calculations for the above processes at various potential ion-exchange sites in the CHA framework indicate that these reactions are thermodynamically feasible. We also find that Pd+ is not formed in the absence of adsorbed water and is readily reoxidized to Pd2+ by trace amounts of O2.