Molecular Dynamics Kinetic Study on the Zeolite-Catalyzed Benzene Methylation in ZSM-5
S. L. C. Moors, K. De Wispelaere, J. Van der Mynsbrugge, M. Waroquier, V. Van Speybroeck, ACS Catalysis 2013 3 (11), 2556-2567.
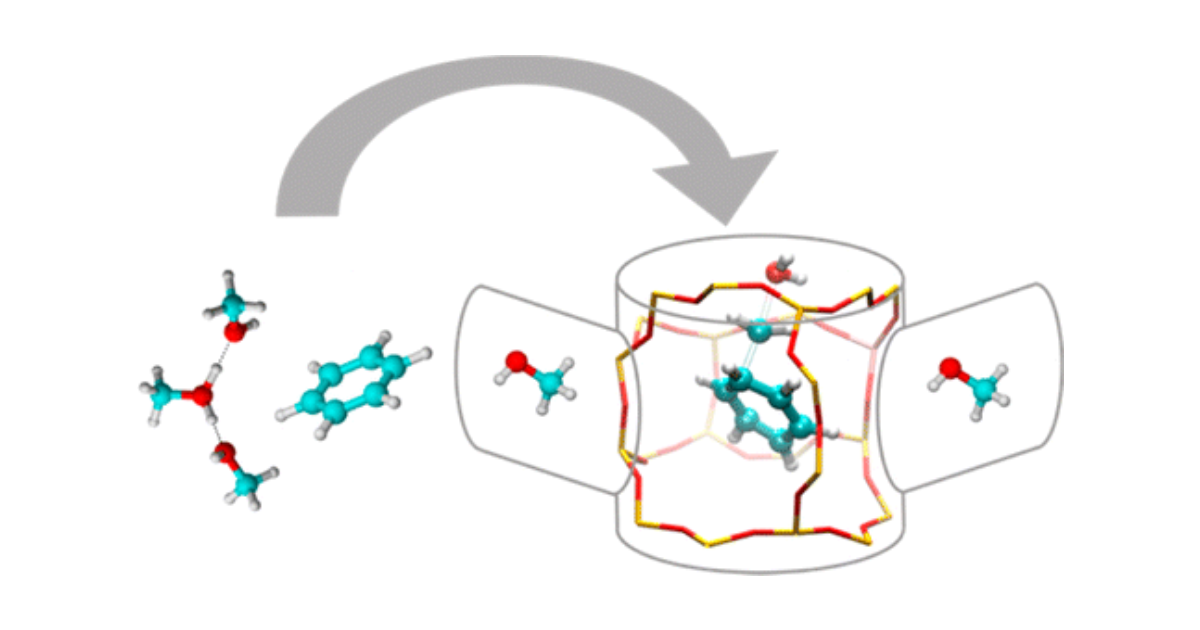
Abstract
The methylation of arenes is a key step in the production of hydrocarbons from methanol over acidic zeolites. We performed ab initio static and molecular dynamics free energy simulations of benzene methylation in H-ZSM-5 to determine the factors that influence the reaction kinetics. Special emphasis is given to the effect of the surrounding methanol molecules on the methylation kinetics. It is found that for higher methanol loadings, methylation may also occur from a protonated methanol cluster, indicating that the exact location of the Brønsted acid site is not essential for the zeolite-catalyzed methylation reaction. However, methylations from a protonated methanol cluster exhibit higher free energy barriers than a methylation from a single methanol molecule. Finally, comparison with a pure methanol solvent reaction environment indicates that the main role of the zeolite during the methylation of benzene is to provide the acidic proton and to create a polar environment for the reaction. The metadynamics approach, which is specifically designed to sample rare events, allows exploring new reaction pathways, which take into account the flexibility of the framework and additional guest molecules in the pores and channels of the zeolite framework. This approach goes beyond the often applied static calculations to determine reaction kinetics.